Snakebites kill between 80,000 and 140,000 people every year. Better antivenom should be a high priority – thankfully new technology can help.
A farmer is working in the rain forest. One day while working her field, she suddenly feels a searing pain in her leg. She sees a snake dislodge its fangs from her calf and quickly slither away. Was it venomous or not? Even if it was a venomous snake that bit her, it might just have been a dry bite, meaning the swelling will go away after a while. The trip to the nearest medical facility will take an entire day and cost her a fortune. She decides to wait and see. After a while the symptoms get worse and she goes to consult her family. Her parents tell her to go to the town’s traditional healer, who once treated her uncle and for a much smaller cost than a hospital would charge. Feeling weak and nauseous when the morning comes around, she finally decides to make the long journey to the hospital. It’s now too late. By the time she arrives the venom has done its damage. Even if she receives the correct antivenom treatment and survives, she will suffer permanent disability and be in for a long and expensive hospital stay. After a few weeks spent recovering in the hospital, she finds herself handicapped, out of work, and in debt.
From the invention of antivenom in 1895 until the 1970s, each decade was looking better than the last. Since then a series of unfortunate events coincided to leave much of Africa without antivenom. Mismanaged government responses and the following market failures caused antivenom production to stagnate resulting in thousands dying unnecessarily every year as a result.
Venomous snakebites kill between 81,000 and 138,000 people each year, and leave another 400,000 with permanent disabilities. This ranks it among the deadliest of neglected tropical diseases, alongside better-known ailments such as typhus and cholera.
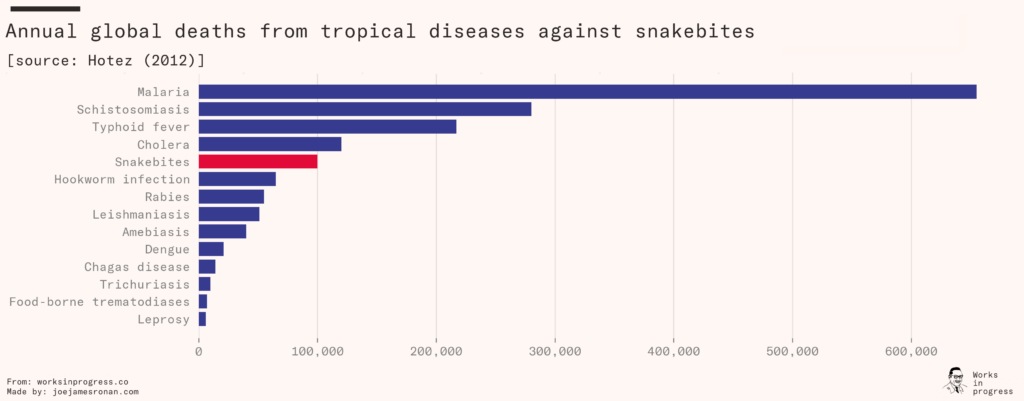
For many years, the number was believed to be much lower. The World Health Organization had previously estimated that only 50,000 died from snakebites each year, and the problem – known as envenoming – was prioritized accordingly. In 2014, an enormous study documenting one million deaths in India concluded with surprising results. They found that 46,000 people were dying yearly from snakebites in India alone, five times more than the WHO had anticipated. The WHO subsequently doubled their global estimate from around 50,000 to their new range of 81,000 to 138,000.
Despite playing host to the world’s most venomous snakes (including the inland taipan, the most venomous animal in the world), Australia averages only two deaths from snakebites each year. This is because deaths from snakebites are a symptom of poverty. A mother in rural India walking barefoot to collect water from the nearby river is much more exposed than an Australian suburbanite surrounded by asphalt.
When a victim is bitten, they begin a race against the toxins spreading throughout their body. To win, they need antivenom. But what is antivenom? It consists of antibodies that disarm the venom’s toxins before they can do the body permanent harm. An Australian is typically a short drive from a well-equipped hospital carrying antivenom in cold storage. Australian doctors and others in the West can use advanced diagnostic equipment to determine the species of snake the patient was bitten by and administer highly effective species-specific antivenom.
An Indian victim, on the other hand, would typically face a long journey to the nearest clinic. For over 34 percent of Indian snakebite victims, it takes more than six hours to receive treatment. If the clinic lacks a cold chain – a coordinated system of temperature-controlled environments – it is limited to using antivenom that can survive room-temperature storage. Without equipment to diagnose the species of snake the patient was bitten by, doctors must use polyvalent antivenom designed to work against multiple species of snake, which is typically more expensive and less effective than species-specific monovalent antivenoms.
Antivenom is no miracle cure and adverse immune reactions to it are common. Patients need to be monitored for up to multiple weeks after receiving antivenom in case of delayed serum sickness, which can be fatal.
Not only is antivenom treatment a dangerous affair; it’s also expensive. The average cost of antivenom alone in sub-Saharan Africa was $124 in 2010, a steep price to pay for the 40 percent of sub-Saharan Africans who live on less than $1.90 per day. And that doesn’t even include the cost of the care or facilities. A survey of 129 Bangladeshi snakebite victims found that over 40 percent had to take out loans to meet the costs. Some had to sell their valuables or useful capital like bicycles to repay the loans. Four victims in the study were forced to pull children out of school and into work to make up for the lost income.
For decades the cost of antivenom in the third world has refused to fall, and in some instances has gone up. In many countries, major antivenom producers are even ceasing production of high-quality antivenom. This has left markets flooded with low-quality products that work less well. In 2017, the World Health Organization rang the alarm bell on what has been dubbed ‘the antivenom crisis’, introduced snakebite envenoming to the list of neglected tropical diseases, and launched a plan to halve deaths from snakebites by 2030.
The arduous history of treating snakebites
“Then the LORD sent venomous snakes among them; they bit the people and many Israelites died.”
Numbers 21:6
Snakebites are no new phenomenon. For millennia humans have sought to prevent bites and cure the bitten.
Throughout the eighteenth and nineteenth centuries, Australian colonists underwent a crash course in preventing and treating snakebites. Snakes were treated as pests to be eradicated. People would go on regular hunts to rid the ‘loathsome reptiles’ from populated areas. One newspaper columnist proclaimed that ‘a natural antipathy and rooted aversion to a brood no less inimical and dangerous to humanity ought to direct us to destroy the serpent’.
There were even several attempts to import mongooses, a natural predator of the cobra, from India to Australia to aid the crusade against snakes. The mongooses did not fare well in the Australian climate. Thus, hopes of eradication died with the mongooses, and there have to my knowledge been no documented attempts to entirely exterminate snakes anywhere in the world.
Historically, those unfortunate enough to be bitten by snakes faced a diverse variety of treatments, few of them effective. No remedy went untested, from chlorinated lime to toad urine. Attempting to prevent the venom from spreading, doctors would apply tourniquets and cut out where the patient had been bitten. They would inject ammonia and other poisons into the patient’s bloodstream, attempting to counteract the venom’s lethal effects. The most well-recognized remedy was to ingest large quantities of brandy. While tourniquets were helpful and alcohol didn’t hurt, most interventions such as ammonia injections did nothing at best and killed patients at worst.
It would take until the late nineteenth century for things to really improve.
In October 1891, heavy rains had caused venomous snakes to seek refuge in the huts of a village in French colonial Vietnam resulting in some 40 bites and 4 deaths. Frustrated, the administrator of the district had a snake charmer catch 19 snakes and sent them live in a barrel to the newly opened vaccine institute in Saigon, led by the French bacteriologist Albert Calmette. Calmette had been studying venoms for over a decade, and was thrilled to receive the surprise shipment of cobras. A year prior, a German team (that would later win a Nobel Prize) had been inoculating rats against diphtheria, a bacterial infection. They learned they could immunize other animals by injecting them with serum from the inoculating rats. A British military doctor stationed in Myanmar had also reported that local snake catchers would tattoo themselves with cobra venom to become immune. With this knowledge in mind, Calmette reasoned that the German team’s approach would work for venoms too.
He injected rabbits with increasing doses of cobra venom until they were receiving and surviving many times the normal dose. When he injected nonimmunized rabbits with lethal doses of cobra venom along with the blood serum from the immunized rabbits, the nonimmunized rabbits would survive.
By 1895 he had improved the technique to produce larger and more potent doses of anti–cobra venom serum in donkeys and horses. The same year one of his snake collectors was accidentally bitten and inadvertently became the first human patient in the world to be treated with antivenom. When the snake collector survived, Calmette knew he had discovered the cure for a killer that had plagued the world for millennia.
Many soon followed Calmette’s example, and antivenom experiments around the world added to the understanding of the benefits and limits of antivenom. By 1905 it had been established that antivenom was not universal, but unique to each species of venomous animal. The twentieth century saw Calmette’s technique used to produce a slew of new antivenoms for snakes and other venomous animals around the world, with enticing branding only the twenties could produce.
While less dangerous than injecting patients with ammonia, receiving early antivenom was not fun either. People’s immune systems rarely responded well to receiving blood serum from a horse. Severe allergic reactions to antivenom were common and could be as life-threatening as the bite itself.
Throughout the early twenties, scientists were experimenting with ways to modify the antiserum after it had been extracted from the host animal. They found ways to remove undesirable impurities and learned to split the antibodies and remove the parts that the human immune system reacted negatively to. They were also discovering they could create polyvalent antivenoms, effective against multiple species of snake, by injecting the host animals with multiple species of snake venom. By 1955, there were more than 22 antivenom producers around the world, with four in Africa and five in Asia. The future of antivenom was looking bright. In just over 60 years antivenom had gone from nonexistence to being produced around the world, each decade bringing superior antivenom compared to the previous, with production increasing on every continent.
The latter half of the twentieth century brought further techniques to improve purity, such as diafiltration and chromatography, steadily bringing down the rate and severity of adverse reactions. The techniques required increasingly expensive equipment to perform, but price was no issue for the increasingly rich Western market. The same could not be said for markets outside of the West, to which companies were struggling to profitably provide antivenom.
The antivenom crisis
In 1970, Africa had three main suppliers of antivenom: a French firm, a German firm, and the South African Institute for Medical Research (SAIMR). But problems were starting to show. The German firm, Behringwerke, made the decision to shut down the entirety of its antivenom production, citing low profitability. Anti-apartheid laws imposed by African governments additionally blocked access to antivenom produced by SAIMR, resulting in erupting black markets across sub-Saharan Africa. Meanwhile the French firm – Sanofi – was repurposing its factories to produce a higher-potency antivenom targeting the higher end of the African market, where profit margins were higher.
This triple whammy would cause critical shortages across Africa throughout the 1990s and 2000s. African governments, already strained by an exploding HIV/AIDS epidemic and desperate to supply their populations with something, would order antivenoms produced in Asian countries targeting deceptively similarly named snakes but that were next to useless for their African counterparts. After switching from FAV-Afrique, Sanofi’s new high-end antivenom, to a cheaper antivenom manufactured in India, which falsely claimed to be effective against African snake species, a Ghanaian hospital reported a sixfold increase in case fatality, to over 12 percent.
A 2017 study testing the efficacy of six antivenoms commonly available in Kenya against the venoms of the most prevalent Kenyan snakes found that not a single one worked. Regional differences between species meant even the ‘gold-standard’ antivenom manufactured by SAIMR needed a much larger dose to be effective against the snakes in Kenya it was thought to protect against.
The mess of poorly tested, mislabeled, and at times outright fraudulent products has resulted in the current predicament: a market for lemons. Confidence in antivenom treatment has fallen. Why go to the hospital for a treatment that doesn’t work when you can go to a traditional healer and get a treatment that doesn’t work for half the price? The market for lemons has led to decreased demand and producers of high-quality antivenom have seen their profitability drop as a result, creating a vicious cycle.
The crisis continues to worsen. Need for antivenom is increasing each year, yet the supply of quality antivenom fails to keep up. A 2011 study found six companies producing antivenom sold in sub-Saharan Africa. These companies produced 410,500 ampoules of antivenom, just enough to treat 96,000 cases. That’s only one third of the total estimated cases on the continent. Their sales totaled just $11 million. The two largest manufacturers, responsible for producing 350,000 of the 410,000 ampoules, were known to use venom immunogens from snake species irrelevant to Africa, a red flag that their product might not work as well as advertised. In 2014, the French producer Sanofi ceased production of its dependable FAV-Afrique, exiting the African antivenom market for good and dealing another blow to the availability of working antivenom. The global antivenom market in 2016 was valued at $1.1 billion and was projected to reach $1.5 billion by 2021. It instead fell to $1.02 Billion.
The difficulties of antivenom production
Why has supplying Africa and other poor regions with working antivenom proven so difficult?
Since its invention, the way we produce antivenom has not fundamentally changed. Around the world snake handlers are manually milking snakes for their venom, injecting it into large animals, typically horses, and tapping their blood to extract the antiserum. This is an expensive procedure that involves keeping horses in stables, keeping snakes in cages, employing animal handlers to milk the snakes, and investing in numerous pieces of technical equipment to tap the blood, extract the antivenom, and purify it.
Moreover, developing countries face a unique set of challenges that make profitable production and distribution of antivenom especially difficult. The lack of cold chains to clinics and pharmacies means manufacturers have to freeze-dry the antivenom so it can be stored at room temperature, which adds an extra step to the manufacturing process and drives up the price.
Regions populated by venomous snakes are usually populated by multiple species. In India, for example, three different species of snake make up the majority of envenoming cases. Clinics in the developing world typically aren’t equipped to accurately diagnose which species of snake a patient was bitten by. To get around this constraint antivenoms must be made polyvalent. This is done by injecting the host animal with venom from multiple snake species, further driving up the cost of production.
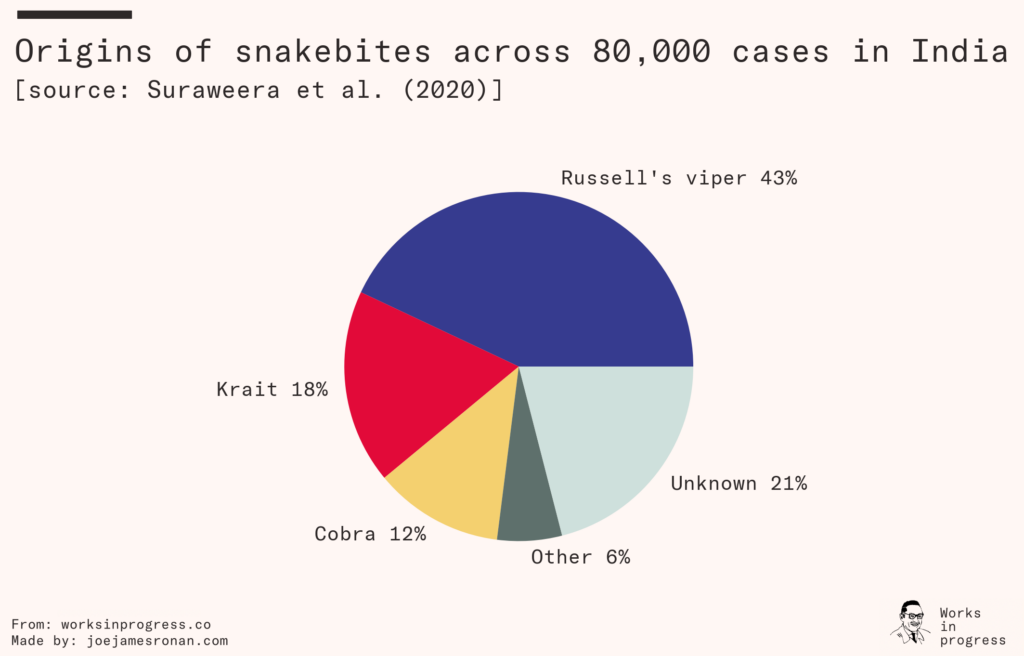
Each species of snake injects a unique blend of toxins when they bite, typically delivering some mix of neurotoxins that destroy the victim’s nervous system, cytotoxins that clot the victim’s blood and cause heart failure, and myotoxins that cause paralysis and necrosis. A bite from the Russell’s viper, common in much of Asia, causes immediate pain and swelling and if left untreated can cause organ failure in anything ranging from a day to two weeks. The black mamba, common across Africa, delivers a venom that can cause the victim to become delirious and collapse, killing the patient in 7–15 hours.
Antivenom targeting a snake species that primarily produces neurotoxic venom will be next to useless against venom that primarily is cardiotoxic. Not only does each species significantly differ, but the composition will differ regionally within each species of snake.
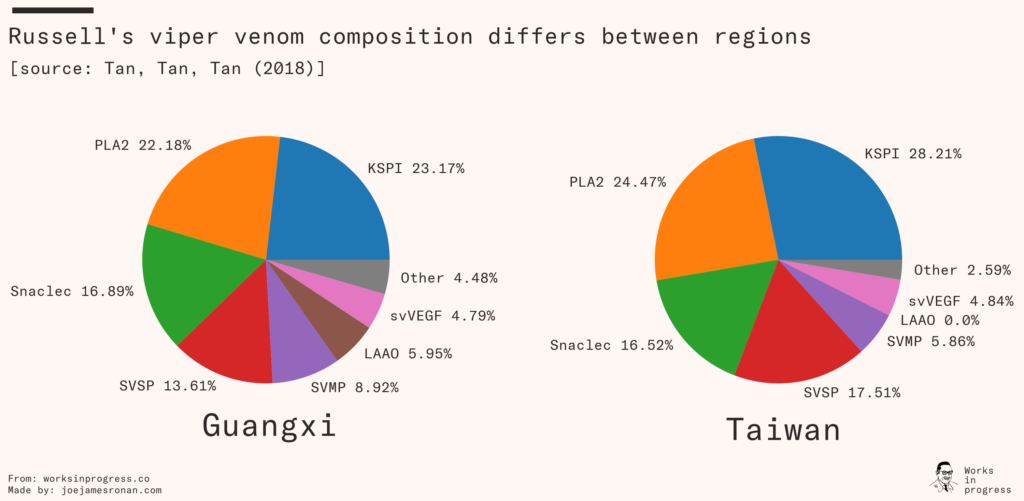
Regional differences explain why even the ‘gold-standard’ polyvalent antivenom was less effective when used on Kenyan snakes, and reveals the difficulty of creating antivenom for large regions. Producing polyvalent antivenoms that verifiably work against the many species of snake across sub-Saharan Africa is a massive undertaking. You need to collect and breed farms of every common species of venomous snake from each region where the antivenom needs to work.
Like most goods, antivenom production is subject to economies of scale. Most estimates suggest that an increase in throughput would radically lower the costs. A projection for African antivenom production estimated that increasing throughput by 50 times would reduce the marginal cost by 95 percent.
If that is true, why hasn’t anyone built massive factories yet?
Even if antivenom production at high throughputs and low prices would be profitable over a longer time horizon, producers have been unwilling to make the large up-front investments necessary to achieve that scale, in no small part due to uncertainty over whether governments and clinics would follow through on promises to buy at those prices. Governments and clinics, on the other hand, have become wary of making large purchase orders after being burned one too many times on promises of cheap antivenom.
Solving the crisis
As long as antivenom remains unaffordable people will continue to die. Either antivenom has to become cheaper to produce or more profitable to sell. Fortunately there are reasons to be optimistic on all fronts.
Firstly, global poverty has been on a steady decline for decades. A wealthy population is one that can afford hospital infrastructure with reliable cold chains and high-quality antivenom. It is also one that can afford protective footwear and undertake other preventive measures.
Secondly, if the studies on scale are accurate, unaffordable antivenom is not a law of nature but a coordination problem to be solved. As part of its 2030 campaign, WHO is facilitating discussions between governments and manufacturers on standards for antivenom efficacy, investments into production, and more.
The antivenom crisis presents a tremendous opportunity for the use of advance market commitments, as pioneered by Nobel laureate Michael Kremer, to incentivize producers to create and scale up production of efficacious antivenoms.
If antivenom producers can be made confident that large investments will pay off, they are more than happy to make them. If governments are confident the antivenom they receive actually works, they are more inclined to buy it. When people start returning from the hospital healthy, happy, and with their wallets intact they are likely to go again and recommend their loved ones do too. In the Bangladeshian survey, most victims and their relatives expressed they would be willing to discontinue traditional healing practices if hospital treatments were easily available.
Economic growth and economies of scale are not the only things working in humanity’s favor. Innovation in how we produce antivenom holds the promise to upend many of the constraints that limit treatment in developing nations.
The promise of new technology
Diagnosing snakebites remains a large issue in not only developing countries, but in middle-income nations such as Brazil as well. Inability to diagnose if a patient needs antivenom before symptoms start to show causes needless death and disability that could have been avoided with timely treatment. Cheaper and better diagnostics would not only improve treatment, but also lower the cost of antivenom as well. If the species of snakebite can be cheaply and rapidly diagnosed, rather than using the expensive and less effective polyvalent antivenoms, it becomes possible to use cheaper-to-produce monovalent antivenom specifically targeting the species of snake the patient was bitten by.
A radical improvement in diagnostics is not going to be achieved by handing out thick books to doctors at local clinics on how to better spot the symptoms. Radical improvement comes from creating a cheap, reliable, and easy-to-use test that eliminates the need for those books in the first place. This is what led a group of Danish researchers to create a lateral-flow test capable of detecting species-specific envenoming long before symptoms set in. Such a test costs cents to produce and distribute. And fortunately for the researchers the world has just gone through a crash course on how to use lateral-flow tests. In 2018 the researchers formed the company Venomaid, which will produce and distribute these tests, making faster, cheaper, and better treatment possible.
Production of synthetic antivenom could drive down the price even further. In 2020 a team of Dutch scientists used stem cells to grow artificial snake venom glands capable of producing crude venom. Scaled up, this makes it possible to entirely eliminate the dangerous and expensive snake-milking process, significantly reducing the costs of antivenom production. In 2018, a team of Spanish and South American scientists produced venom antibodies in gene-modified plants, which similarly would remove host animals from the production process.
Synthetically created antivenom would eliminate the most expensive and difficult-to-automate steps of antivenom production and could conceivably bring down the marginal cost by orders of magnitude. There are now start-ups looking to make this a reality. An example of such a startup is Venomyx, which claims it will be able to produce antivenom grown in bacteria that is ten times more potent than its animal equivalent, has fewer side effects, is shelf-stable, and is cheaper to produce.
Innovations like these could put us back on track to cure snakebites. Yet despite their promise, the field remains poorly funded. Investment into venomics research makes up just two percent of the snakebites budget in the WHO’s 2030 plan. Relying on venture capital instead, start-ups in the field are urged to focus on richer markets with high profit margins rather than where their products would do the most good. This is not to point blame at venture capitalists who are providing much needed funding where there otherwise would be little, but to emphasize that nonprofit funding in the space could shift priorities for the better.
The philanthropic case for even just scaling up traditional antivenom production is promising. A study estimating the cost-effectiveness of subsidizing antivenom treatment found the cost per death averted similar to the top charities recommended by the charity evaluator Givewell. If the cost of antivenom could be brought down one order of magnitude, antivenom treatment has the potential to be among the most cost-effective causes in the world.
Promising new producers are entering the field with new clinically proven products. The faster they can scale up and meet demand the more suffering can be averted. While the profitability of these new producers is yet to be proven, this shouldn’t dissuade the risk-willing philanthropist for whom the return on investment is measured by lives saved cost-effectively.
Decades of government mismanagement and failure of the international aid community to intervene resulted in a flatlined antivenom market and caused needless suffering. With renewed interest from governments and international organizations to create favorable market conditions, innovators creating new treatments, and new manufacturers scaling to meet the demand, one can hope the 2014 exit of Sanofi marked the peak of an antivenom crisis that is now being banished for good.
In time, the countless deaths from snakebites will be a relic of a distant past. Let’s strive to bring about that time sooner rather than later.