Synthetic diamonds are now purer, more beautiful, and vastly cheaper than mined diamonds. Beating nature took decades of hard graft and millions of pounds of pressure.
A diamond – from the Greek ἀδάμας (adámas), meaning unconquerable – is a three-dimensional cubic or hexagonal lattice of carbon atoms. As its bonds are strong and its atoms packed closely together, diamond is the hardest natural material and the least compressible. Diamonds have high thermal conductivity and high electrical resistivity, but can be combined with small amounts of nitrogen, phosphorus, and boron and made into semiconductors. A diamond’s surface does not easily stick to other materials, but moves smoothly against them. Diamonds are chemically inert and not toxic to living tissue. In their pure form, diamonds are colorless and have a high dispersion of light, but the presence of certain impurities can add magnificent color to diamond gemstones.
In nature, it takes billions of years to form a diamond. Most of the diamonds nature produces are too impure for jewelry or high-tech industry, and extracting them is costly and dirty. In the lab, diamonds can be made faster, purer, and cheaper, overcoming these problems and making possible new uses for diamonds that were previously unattainable.

Subscribe for $100 to receive six beautiful issues per year.
Scientists first manufactured diamonds in laboratories in the 1950s, imitating the conditions under which diamonds were produced in nature. The diamonds produced were initially small and impure and so useful only in low-tech industrial products such as abrasives and lubricants. Since then, diamond manufacturing technology has progressed: the generation process has become more controlled, new methods have been invented, and better catalysts have been discovered. Diamonds grown in the lab are now cheaper than mined diamonds and have superior physical, optical, chemical, and electrical properties. Consequently, they dominate the industrial market. In the past decade, diamond manufacturing technology progressed so much that it is now possible to mass-produce jewelry-quality diamonds in the lab. These lab diamonds are cheaper and more beautiful than mined diamonds. A perfectly cut, flawless lab diamond costs a fraction of the price of a mined diamond of lesser quality.
Lab diamonds are a testament to the principle that what nature can do, man is capable of doing better.
How diamonds are made
In 1773, in a series of experiments in the gardens of the Louvre, the French chemist Antoine-Laurent de Lavoisier placed diamonds into a glass bell jar and then immersed the base of the jar in water. Using a large, biconvex lens that measured 89 centimeters and weighed over 70 kilograms – the Palais-Royal burning glass – Lavoisier would concentrate the sun’s rays onto a diamond. Within minutes, the diamond on which the lens had been focused would evaporate completely; the others would blacken and lose mass.
In his Memoir on the Destruction of the Diamond by Fire Lavoisier recounts that these experiments had been conducted in ‘clear and serene’ weather, but on 14 August 1773 a light fog reduced the sun’s power. In the more moderate heat, the diamonds in the jar appeared ‘precisely as if they had been coated with black smoke in the flame of a lamp’, causing Lavoisier to conclude that diamond is susceptible to be reduced to coal – charbon – in certain circumstances. Overturning the jar and pouring inside limewater before the air escaped, Lavoisier observed that a chalky substance was precipitated when both diamonds and coal were burned. However, he did not conclude that diamond and coal are the same substance, only that both are combustible bodies.
In his 1789 Elementary Treatise on Chemistry, Lavoisier proposed that there exist basic chemical building blocks, of which carbon – from the Latin carbo, meaning coal – is one. He would not live long enough to discover that diamond is an allotrope (a different physical form of the same element) of carbon.
In the senseless bloodshed that characterized the French Revolution, Lavoisier, who had also been a tax farmer, was accused of adulterating tobacco and defrauding the state. He was guillotined on 8 May 1794. An apocryphal story holds that in response to an appeal to spare his life so that he could continue his experiments, the Revolutionary judge Jean-Baptiste Coffinhal declared that ‘The Republic needs neither scholars nor chemists; the course of justice cannot be impeded’. Coffinhal himself would be executed for his brutality three months later, but the damage had already been done. As Lavoisier’s colleague, the mathematician and astronomer Joseph-Louis Lagrange, mourned, ‘It took them only an instant to cut off this head, and one hundred years might not suffice to reproduce its like’.
Instead, the discovery that diamond is carbon would be made by the English chemist Smithson Tennant, in 1796. Tennant heated ‘two grains and a half’ (162 milligrams) of diamonds in a closed gold tube containing niter (potassium nitrate, KNO3). When heated, the potassium nitrate released oxygen, increasing its concentration, so that the diamonds burned faster, and at a lower temperature. Tennant kept the gold tube ‘in a strong red heat’ for an hour and a half, after which the diamonds were completely burned. The reaction produced ‘fixed air’ (carbon dioxide). Extracting and measuring the carbon dioxide produced, Tennant noted that it had occupied the space of ‘a little more than 10.1 ounces [354 milliliters] of water’.
In the Memoirs of the Royal Academy of Sciences for 1781, Lavoisier had published the results of a series of experiments in which he identified that ‘carbonic acid’ (carbon dioxide) contains 28.22 parts of ‘charcoal’ (carbon) for every 71.78 parts of ‘acidifying principle’ (oxygen) and, at similar temperatures and pressures to the air in Tennant’s laboratory, determined its weight to be 0.695 Parisian grains per cubic Paris inch (1.86 grams per liter). Using these measurements, Tennant computed that the carbon dioxide produced by burning an equal weight of charcoal ‘ought to occupy very nearly the bulk of 10 ounces [352 milliliters] of water’.
On the basis that the quantity of carbon dioxide produced from burning equal weights of diamond and charcoal ‘does not differ much’, Tennant concluded that diamond ‘consists entirely of charcoal’. This astonishing result would be met with skepticism for two decades, until other scientists reproduced his results.
Tennant had demonstrated that diamond is an allotrope of carbon, but diamond’s method of formation remained unknown. Motivated by the prospect of discovery and the allure of riches, nineteenth-century alchemists tried to turn charcoal into diamond by various means, including evaporation, explosions, and intense heat. None met with success.
The most important of these researchers was the French chemist Henri Moissan. In 1886, Moissan succeeded in isolating the chemical element fluorine by means of electrolysis; for this, and for his research into high-temperature chemistry, he would win the 1906 Nobel Prize in Chemistry. Considering the problem of synthesizing diamonds, Moissan tested different forms of carbon – burnt sugar, charcoal, gas carbon, and carbon black – under the high heat of an electric arc furnace. In each of these experiments, the carbon would be converted into graphite, a different allotrope of carbon.
Moissan examined the geological records from sites where diamonds had been discovered – the alluvial sands of Brazil, the diamond pits of southern Africa, and the Canyon Diablo meteorite in Arizona – and made two important observations. First, he noticed that the diamonds of southern Africa, which were contained in rock that had been ‘thrust up . . . from the deeper layers of the earth’, must have formed deep underground – ‘and thus pressure must have played a part at the moment of formation’.
Second, the diamonds that were discovered had not been attached to the ground in which they were buried; instead, they appeared as if they had formed ‘in the midst of a liquid’. Carbon, including diamond, dissolves easily in iron, and iron is frequently found near diamond sites, including the Canyon Diablo meteorite, where two small diamonds had been found in the middle of an iron mass. ‘In this case’, Moissan noted, ‘nature seems to have been caught in the act’.
The Canyon Diablo meteorite suggested how diamonds might form. Molten iron that contains dissolved carbon might cool suddenly, ‘due to some cause or other’, causing it to contract ‘violently’, pressurizing the dissolved carbon to crystallize into diamonds. To replicate this process in his lab, Moissan heated crucibles containing iron and burnt sugar to 3,000°C and then quenched them in cooler substances: water, iron filings, and molten lead. In the residues, under a microscope, he discovered dense fragments of carbon, some black, others transparent, that scratched rubies, combusted in oxygen, and had ‘a very distinct crystalline appearance’. Moissan measured the relative density of these fragments to be 3.5 (compared to water) and observed that when burnt in oxygen at 900°C they yielded 3.666 grams of carbon dioxide per gram of substance. ‘These properties’, he declared, ‘are possessed by the natural diamond alone’.

Moissan would go to his death in 1907 believing that he had synthesized diamonds. Other chemists, including Henry Louis Le Chatelier, were skeptical and his experiments did not replicate. The German chemist Otto Ruff and the English engineer Sir Charles Parsons separately believed that they had reproduced his results, only to repudiate their findings after more careful examination. Moissan’s widow would later tell Parsons that she believed one of Moissan’s assistants had introduced natural diamond fragments into the experiments ‘in order to please the old man’. Nonetheless, despite his failure, Henri Moissan was correct to believe that diamonds could be synthesized under a combination of high pressure and high temperature, catalyzed by a metal solvent.
High pressure, high temperature
In 1950, the General Electric Research Laboratory in Schenectady, New York, assembled a consortium of chemists, physicists, and engineers to form Project Superpressure, an effort to synthesize diamonds in the lab. General Electric, the industrial conglomerate founded by the inventor Thomas Edison, had a strong tradition of industrial research and the cost of importing diamonds, used to draw out the tungsten filament wire in light bulbs, was a serious concern. To support its research, General Electric commissioned a hydraulic press that cost $125,000, stood two storeys high, and was capable of pressing 1,000 tons.
Four years of intense experimentation followed, during which Project Superpressure exhausted all of its original research budget and two additional funding allocations. On the third occasion that the project manager asked for more money, General Electric’s research managers, having seen no tangible results and skeptical of future success, almost unanimously voted to discontinue their support. Guy Suits, General Electric’s director of research, overruled them and approved the funds. Shortly after, Project Superpressure would make its breakthrough.
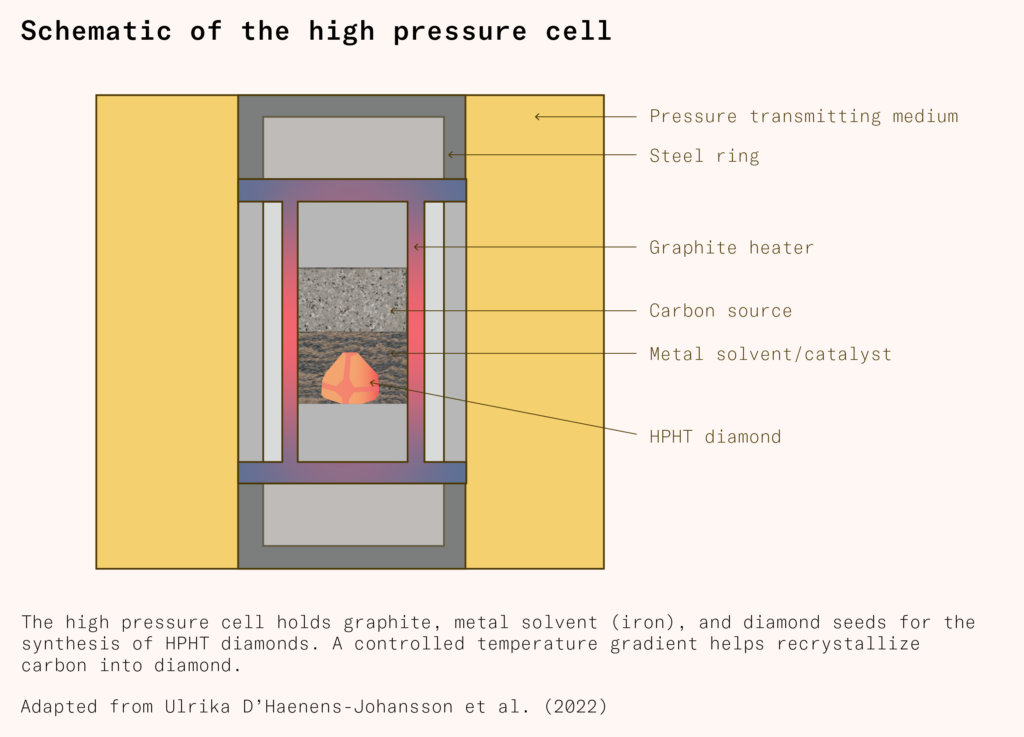
On 16 December 1954 chemist and Project Superpressure team member Howard Tracy Hall placed two diamond seed crystals into a graphite tube with iron sulfide, capped with tantalum disks. The tube’s graphite would act both as a carbon source and, when electric current was applied to the tantalum disks, a resistance heater. He then placed this cylindrical device into a pressure chamber of his own design. Hall’s pressure chamber, now known as a belt press, consisted of two opposing tapered anvils that compressed the reaction cell from above and below, with the sides supported by prestressed steel bands.
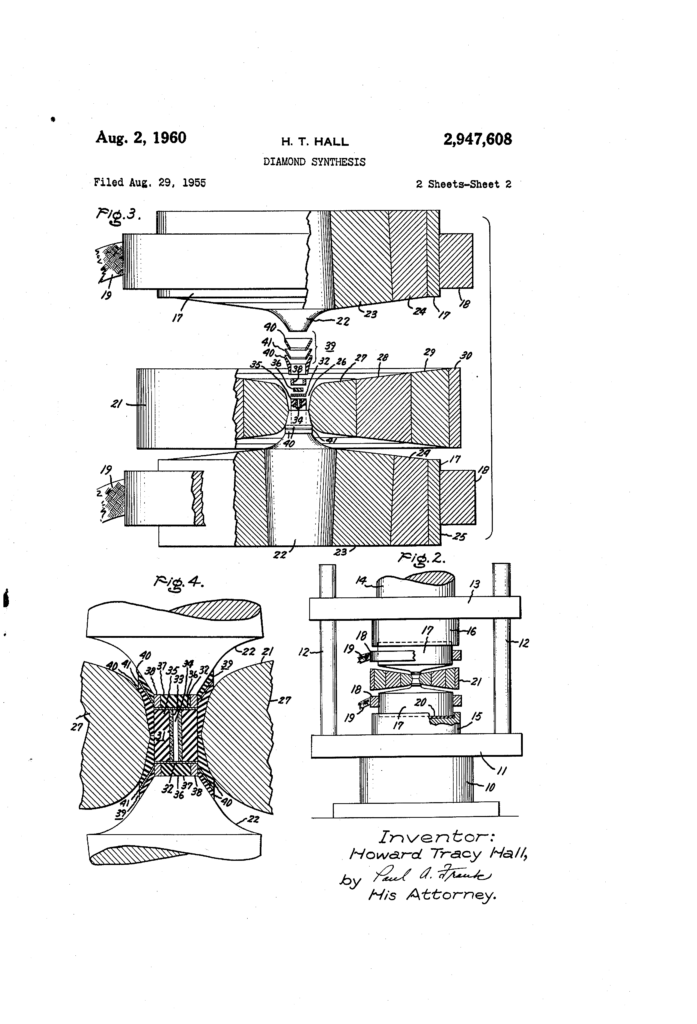
Hall describes how when he envisioned this device, his colleagues ‘felt negatively about it’. His proposal to build a prototype, which would have cost General Electric less than $1,000, was rejected and he was refused time in the machine shop to build it. ‘I fretted about this for a time’, he wrote, ‘and then decided on a sub-rosa solution. Friends in the machine shop agreed to build the Belt, unofficially, on slack time. This took several months. Ordinarily, it would have taken only a week.’
A practicing Mormon, whose church and large family left him little time to socialize with his colleagues, Hall attributed the refusal to build his design and other slights to religious prejudice. When his prototype belt apparatus proved capable of attaining high pressures and high temperatures, Hall requested that its critical components be reconstructed in carboloy (cobalt-cemented tungsten carbide). Once again, his request was refused and it was not until his former supervisor intervened that he obtained permission to buy the carbide components.
To further compound Hall’s sense of injustice, demand for Project Superpressure’s thousand-ton press was so high that Hall’s improved pressure chamber was ‘relegated’ (in his words) to an ‘ancient’ press, dating from the turn of the twentieth century, that was only capable of pressing 400 tons and still ran on water pressure. Hall would later describe how this press ‘leaked so badly that rubber footwear, mop, and bucket were standard accessory equipment’.
Using this antique press, Hall managed to compress his pressure chamber to ten gigapascals (about 100,000 times the pressure of the atmosphere) and to heat the reaction chamber to 1,600°C. The experiment ran for 38 minutes. Hall had created diamonds:
I broke open a sample cell after removing it from the Belt. It cleaved near a tantalum disk used to bring in current for resistance heating. My hands began to tremble; my heart beat rapidly; my knees weakened and no longer gave support. My eyes had caught the flashing light from dozens of tiny triangular faces of octahedral crystals that were stuck to the tantalum and I knew that diamonds had finally been made by man.
General Electric reproduced Hall’s results 20 times over the next two weeks and on 15 February 1955 announced to the rest of the world that it had created the first diamonds in the lab. In its press release, it implied that the diamonds had been created in their new thousand-ton press. Hall’s reward was a modest salary increase – from $10,000 to $11,000 per year – and a ten-dollar savings bond. He resigned from General Electric to become a full professor at Brigham Young University.
The diamonds that Hall’s process produced were tiny, measuring a few thousandths of a millimeter in diameter: just enough to glint. Diamonds of this size are too small for jewelry, but extremely useful in industry – at the time, they were (and still are) used for sawing, grinding, and polishing metal, drawing wire, and stamping precision components. General Electric’s industrial diamonds initially cost more than mined diamonds, but they quickly proved to be superior. Unlike in nature, the diamonds’ growth was precisely managed, and their shape and regularity could be made to order.
Hall, meanwhile, was forbidden from disclosing details about the belt press he invented, or using it to pursue further research into high-pressure chemistry, because of a secrecy order imposed by the United States Department of Commerce. During the Second World War, the supply of diamonds had been a source of anxiety for both the Allied and Axis powers. The United States, which did not have a domestic supply of diamonds, was dependent on the De Beers cartel for the diamonds necessary for its industrial production, and the business model of De Beers was to drive up prices by artificially restricting the world’s supply.
A 1944 memorandum to the United States attorney general declared that ‘the United States is paying monopoly prices for an essential material needed in wartime production’ and that if De Beers were an American company ‘there would be no question’ that it had violated antitrust laws. De Beers was headquartered in London and did not maintain a presence in the United States, so there was little the Justice Department could do. In an effort to maintain American technological superiority during the Cold War, the Commerce Department desired to prevent other countries from obtaining diamond-making technology, even if it meant restricting research within the United States too.
Hall attempted to get around this problem by designing a better press. The belt press invented at General Electric transmitted pressure to the reaction cell on one axis only; he determined it could be improved by applying pressure from multiple directions. In 1957, Hall built a tetrahedral press that consisted of four triangular-faced anvils that pressured each of the sides of a tetrahedral reaction cell, and submitted a paper describing its design to the Review of Scientific Instruments.
The Commerce Department placed a secrecy order on this design too and Hall was obligated to write to every person who had seen the tetrahedral press or requested a description of the device to inform them of this directive. ‘In exasperation, I considered giving up the field of high pressure’, Hall would later write. Many of the scientists to whom Hall communicated this directive thought the secrecy order was outrageous and complained to the Commerce Department. Other government agencies agreed and soon after, the Defense Department ordered the Commerce Department to abandon the secrecy orders on the belt and tetrahedral presses.
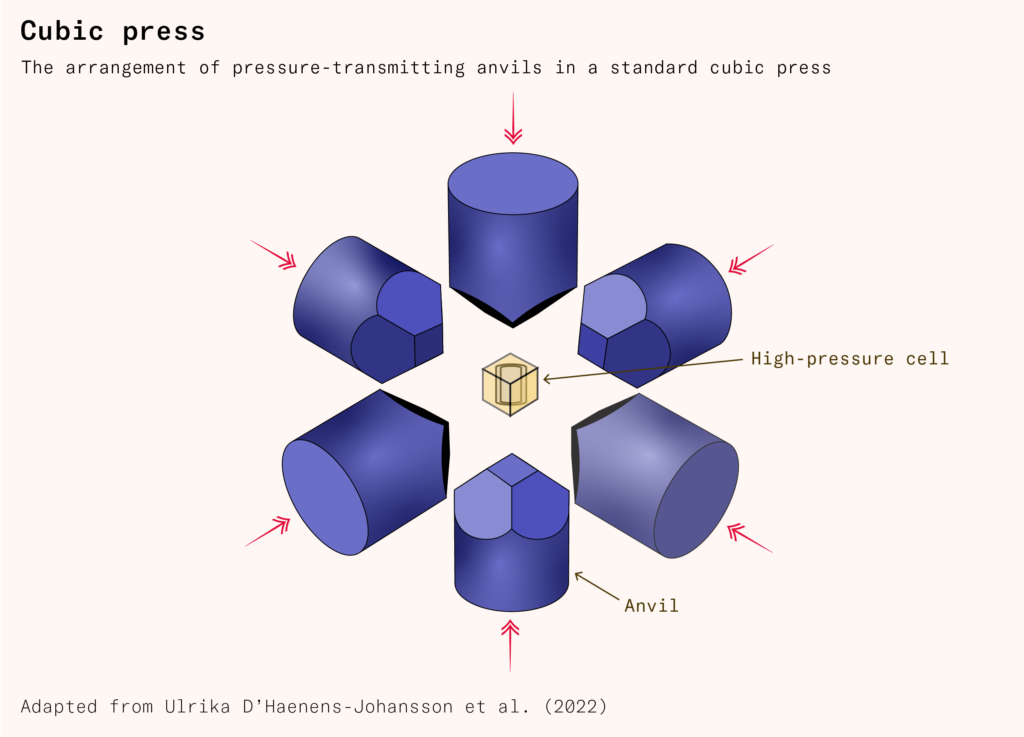
The tetrahedral press was an improvement on the belt press, but more efficient still was the cubic press, which Hall, like other industrialists making diamonds around the same time, would find more practical. A cubic press uses six hydraulically powered anvils to put pressure on the six faces of a cubic reaction cell from each side. Compared to a tetrahedron, a cube has a smaller surface area to volume ratio. Because pressure is force divided by area, this means that the cubic press can achieve the pressures required to grow diamonds with less force.
Scientists at the Siberian branch of the Academy of Sciences of the Soviet Union pursued a different method of manufacturing diamonds under high pressure and high temperature. Instead of using a hydraulic press, the Soviets made use of a split-sphere apparatus. This consists of a cylindrical reaction cell that is surrounded by six inner anvils that fit together to form an octahedron. These inner anvils are, in turn, surrounded by eight outer anvils that fit together to form a sphere. Surrounding the sphere is a thin reservoir. A compact pressure pump injects hydraulic oil into the reservoir, pressuring the sphere from all directions at once. This causes the anvils to squeeze together, magnifying the pressure transmitted to the reaction cell at the center of the sphere (as the force is concentrated onto a smaller area). A graphite heater in the reaction cell provides the necessary heat.
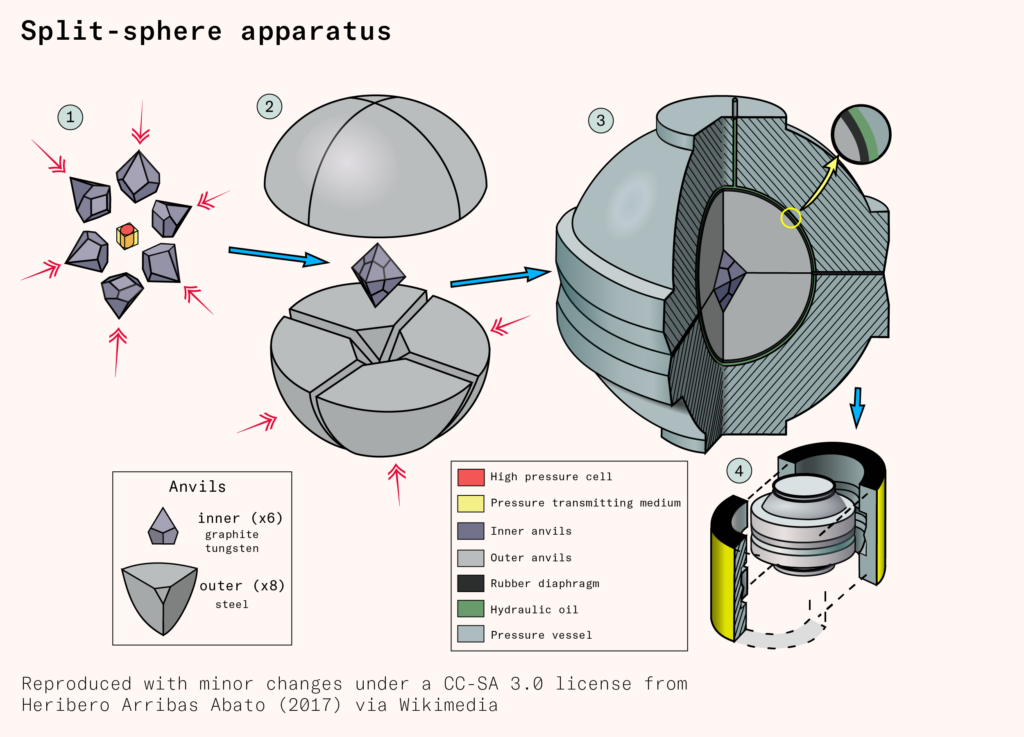
Though the belt press, the cubic press, and the split-sphere apparatus have different pressure-delivery mechanisms, the chemical reaction that occurs in the high-pressure cell is the same: graphite is converted into diamond under high pressure and high temperature, catalyzed by a metal solvent and a small diamond ‘seed’ on which the new diamond precipitates.
Carbon atoms have four electrons in their outermost shell. At low pressures and temperatures, graphite – a solid that consists of two-dimensional sheets – is the most stable phase of carbon. In graphite, each carbon atom is covalently bonded to three other carbon atoms; the fourth electron is delocalized and free to conduct electricity. Graphite sheets are weakly held together by van der Waals forces: repulsions and attractions caused by fluctuations in the electron density of each atom.
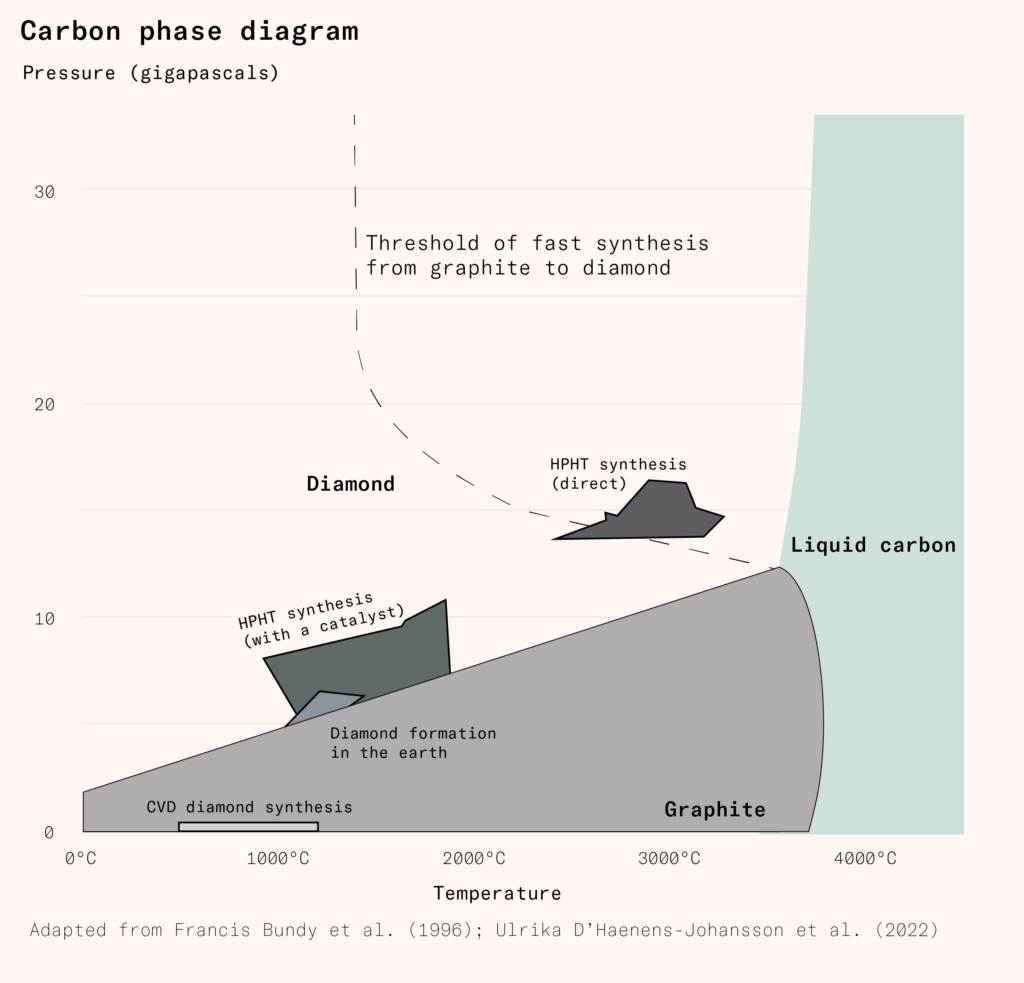
Under high pressure, diamond is the most stable form of carbon: each carbon atom is covalently bonded to four others, creating a tightly packed three-dimensional lattice. However, because the binding energy between carbon atoms is high, to convert graphite to diamond requires high temperatures. When graphite is heated to 3,000°C under 15 gigapascals of pressure, its molecular structure changes to the tetrahedral shape characteristic of diamonds within seconds. Maintaining an even temperature and pressure is difficult under these conditions and so the diamonds produced tend to be small – measuring, at most, a few millimeters – and gritty. The cost of the equipment required to produce the necessary heat and pressure is prohibitively expensive.
Diamond makers use metal solvent catalysts to reduce the pressure and temperature required. In the reaction cell, diamond seeds, graphite, and a transition metal solvent such as iron are heated to 1,300°C–1,800°C and five to seven gigapascals of pressure are applied. The molten metal dissolves the graphite, breaking it down into individual carbon atoms.
At high temperatures, the metal dissolves more carbon atoms. When the temperature falls, this is reversed: the carbon atoms are now supersaturated in the solvent and some of them precipitate out. Diamond manufacturers deliberately encourage this process by keeping the diamond seeds at a lower temperature than the metal, so that carbon atoms precipitate on their surface, growing them into larger diamonds. This process takes weeks, but compared to direct synthesis it is easier to control. The diamonds produced are larger and purer and have a more consistent, single-crystal structure.
When making diamonds in a lab, the most common impurities to control are nitrogen and boron. These elements sit on either side of carbon on the periodic table and, being roughly the same size, are easily incorporated into a diamond’s lattice. Nitrogen colors diamonds yellow; boron colors them blue. Both are ubiquitous in the Earth and in the lab and it is difficult to exclude them from the carbon source, the metal solvent, and the reaction cell’s components. To stop nitrogen and boron from being absorbed into the diamond, manufacturers use chemical absorbers (called getters) that react with them. Aluminum, titanium, and zirconium are common choices for both elements. When people want yellow or blue diamonds, nitrogen or boron can easily be added during production.
Pink diamonds need a few more steps. They are produced by introducing nitrogen into the reaction, irradiating the resulting yellow or brown diamond, and then heating the diamond. This process causes the lattice to rearrange itself so that, throughout the diamond, single nitrogen atoms are located next to single gaps in the lattice. These defects are called nitrogen-vacancy centers and they produce a pink hue. In quantum computing, pink diamonds have been proposed as a means of storing and manipulating quantum information at room temperature.
While diamonds were first produced in the lab in the 1950s, until the 2010s they were too small and too impure for jewelry. What made gem-quality lab diamonds possible was the meticulous investigation of the behavior of carbon at high pressures and high temperatures, a better understanding of the role of catalysts and getters, more precise control of the temperature gradients in the reaction cell, and competition from a new method of manufacturing diamonds.
Chemical vapor deposition
During the 1950s, while Project Superpressure was recreating the high-pressure, high-temperature conditions under which diamonds form in the Earth, scientists at industrial laboratories in the United States and at the Academy of Sciences of the Soviet Union in Moscow investigated a different technique for producing diamonds, not inspired by nature: chemical vapor deposition.
With chemical vapor deposition, a solid material is deposited from a vapor that is undergoing a chemical reaction. As a manufacturing technique, it is as old as the cavemen, who would use soot from combustion for painting. To make diamonds using this process requires heating carbon to such a high temperature that it becomes a gas of isolated atoms, and then coaxing it to crystallize into a diamond structure as it cools.
Graphite is the most thermodynamically stable phase of carbon at low pressures, but it is only slightly more stable than diamond. Because of this, whether the carbon gas condenses as diamond or graphite hinges on how fast the diamond and graphite crystals form and grow.
The first successful effort to produce diamonds using chemical vapor deposition occurred at Union Carbide. According to a patent application filed by research scientist William Eversole in 1958, when a carbon-containing gas (either methane, CH4, or carbon monoxide, CO) is heated to 900–1,100 degrees celsius at low pressure in the presence of diamond seed crystals, ‘diamond is deposited at a much faster rate than is black carbon [i.e., graphite]’. At the time, this was surprising. As the patent explains, ‘This is unexpected in view of the fact that thermodynamically, graphite is more stable than diamond’.
Periodically, the diamonds had to be cleaned: further growth would be ‘hampered by the accumulation of black carbon’. This could be done by exposing the diamonds to hot, pressurized hydrogen (H2), which removes graphite faster than it removes carbon. Unfortunately, the rate of diamond growth in this initial discovery (~0.01 micrometers per hour) was extremely slow and so the process could not be commercialized.
In the 1960s, the American chemist John Angus started an experimental program to investigate chemical vapor deposition diamond synthesis at Case Western Reserve University. Angus recounts that ‘the anonymous [National Science Foundation] reviewers were often scathing in their assessment of low-pressure diamond synthesis proposals, usually based on a misapplication of the second law of thermodynamics’ but the Defense Department were more sympathetic to high-risk proposals, ‘especially if they included, “The Russians are doing it”’.
In their experiments, both the Soviets and the Americans were forced to alternate diamond growth cycles with graphite-cleaning cycles. Angus’s group found that if they broke the H2 used in cleaning into atomic hydrogen, the reaction proceeded much faster. At a conference in Ukraine in 1971, John Angus discussed these results with the Russian chemist Boris Derjaguin. Shortly after, the Soviets stopped publishing.
What the Soviets discovered was that by using atomic hydrogen during the growth phase, they could inhibit the formation of graphite in the first place. Furthermore, the hydrogen gasified and thus removed any graphite that did manage to form. This both eliminated the need for a separate cleaning phase and accelerated diamond growth. While the Soviets would publish this result in 1978 and 1981, national security considerations prevented them from describing their experimental setup in sufficient detail for others to replicate their results.
Despite this secrecy, the results of these experiments attracted the attention of scientists at the Japanese National Institute for Research in Inorganic Materials, who began an intensive program of research into the role of atomic hydrogen in the chemical vapor deposition synthesis of diamonds. Using hot tungsten filaments, microwaves, and electric arcs to break hydrogen gas (H2) into atomic hydrogen (H), these researchers – principally Mutsukazu Kamo, Seiichiro Matsumoto, and Yoichiro Sato – discovered how to grow diamonds at rates of several micrometers per hour, two orders of magnitude faster than Eversole.
Crucially, this research was done in the open: the growth methods were published in sufficient detail that other scientists could easily reproduce and extend their work, and the laboratory welcomed visitors. A large number of companies, universities, and research institutes in Japan, the United States, and Europe entered the field. Having been dismissed as being physically impossible two decades before, the manufacturing of diamonds using chemical vapor deposition had become the subject of intense research and development worldwide.
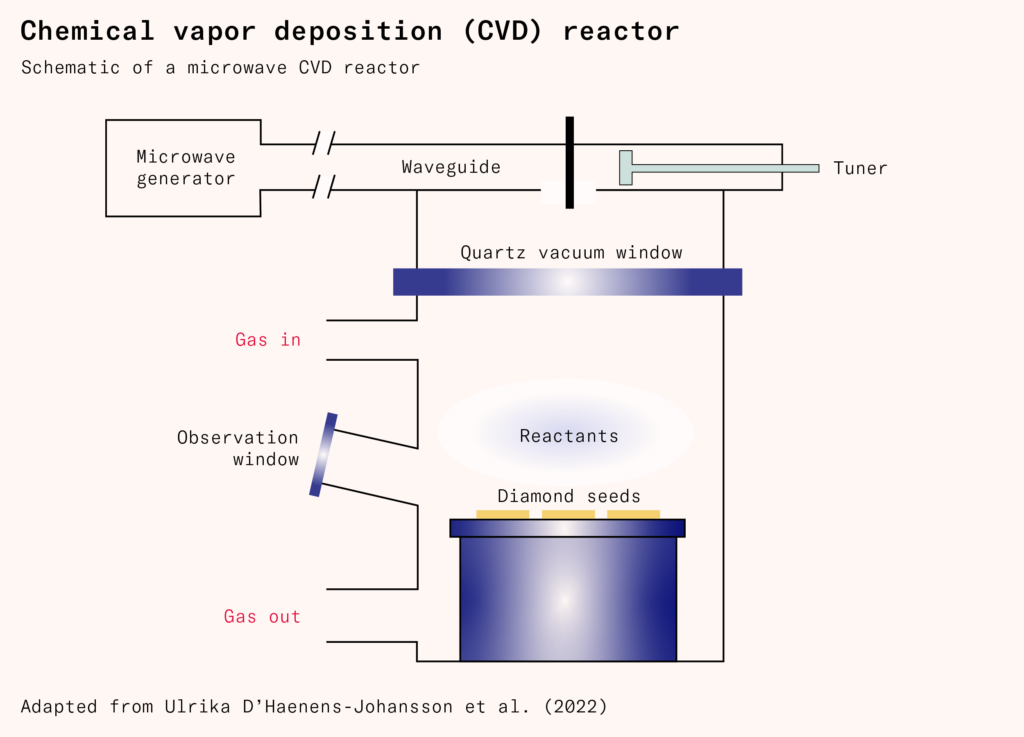
In a modern chemical vapor deposition reactor, multiple diamond seeds are placed in a vacuum chamber with the reactant gasses – mostly hydrogen (H2) and a small amount (generally less than one percent) of methane (CH4). The diamond seeds are heated to 800°C–1,200°C. Microwaves are then used to heat the hydrogen gas (H2) to 2,000°C–5,000°C, breaking its molecules into atomic hydrogen (H).
Some of these unpaired hydrogen atoms will react with the carbon atoms on the surface of the diamond, creating a hydrogen-terminated surface that prevents the diamond crystal from reconstructing itself as graphite. However, because other unpaired hydrogen atoms will also abstract hydrogen from the diamond’s surface, not all of the carbon atoms will be terminated, leaving sites for other carbon atoms to be added.
At the same time, atomic hydrogen will react with the methane, stripping away a hydrogen atom and leaving behind a methyl radical (CH4 + H → CH3 + H2). The carbon atom in the methyl radical (CH3) contains an unpaired electron. Some of these methyl radicals will react with unterminated carbon atoms on the diamond’s surface, growing it. The length of time required depends on the intended size; for gem-sized diamonds, it can take several weeks.
A major advantage of chemical vapor deposition is that the reaction chamber is not subject to extreme pressure, and so the formation of the diamond is easier to monitor and to control. Chemical vapor deposition reactors are also less capital intensive than the presses required for high-pressure, high-temperature synthesis.
Manufacturers can engineer chemical vapor deposition diamonds to precise physical, mechanical, thermal, and optical specifications by varying the temperature, pressure, and duration of the reaction and by adding desired impurities to the reactant gas. Improvements such as slightly increasing the pressure in the reaction chamber, using higher-powered microwaves, and adding a small amount of nitrogen and oxygen to the catalyst have made it possible to grow large, gem-quality diamonds.
Identifying a lab diamond
In their pure form, lab diamonds and mined diamonds are physically, chemically, and optically identical. Both consist of three-dimensional lattices of carbon atoms. To tell them apart, trained gemologists look carefully for impurities included during their formation, and for growth patterns that provide clues about their development. Diamond-manufacturing technology has become so good that lab diamonds can be made to be purer than diamonds formed in the Earth. At the upper end of the market, it is not possible to tell the difference between lab diamonds and mined diamonds with the naked eye.
Mined diamonds almost always contain nitrogen. In large quantities, this colors them yellow or brown; for colorless diamonds, this impurity can be detected using an optical spectrometer. Nitrogen-free diamonds that are formed in the Earth are extremely rare, and expensive. Conversely, nitrogen is almost always excluded from lab-grown diamonds.
High-pressure, high-temperature diamonds can include traces of the metallic catalysts used to make them, particularly if the reaction is not carefully controlled. These can be identified using a microscope, and at high concentrations, iron and cobalt impurities can even be detected using a strong magnet. Chemical vapor deposition diamonds sometimes incorporate graphite, which appear as little pinpricks, comets, or flat clouds.
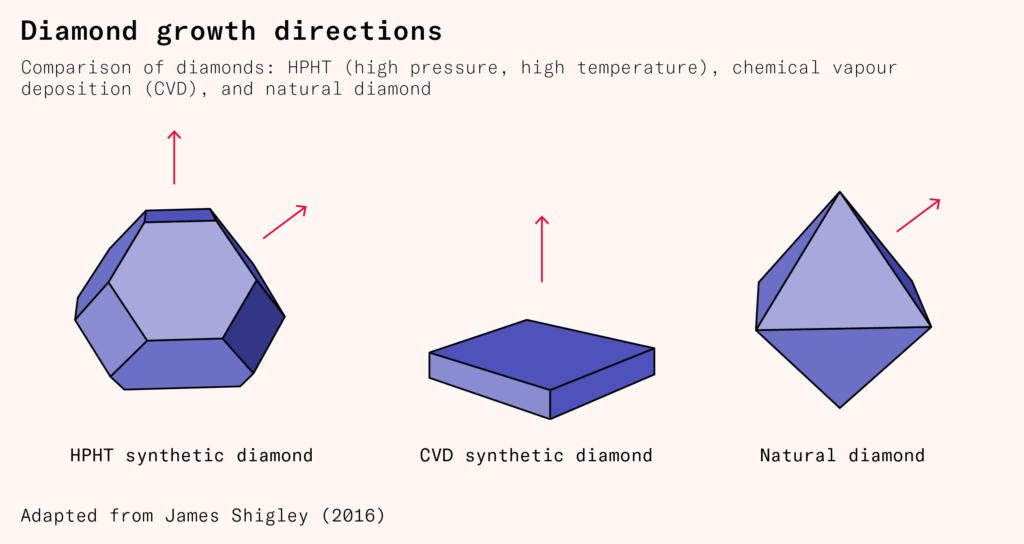
High-pressure, high-temperature diamonds are subject to roughly uniform pressure during manufacturing, leading them to grow in both the cubic and octahedral directions. Even after being cut and polished, some evidence of these growth directions remains in the form of tiny grain lines. Chemical vapor deposition diamonds, deposited layer by layer, are cubic in form. Diamonds produced in the Earth are subject to uneven pressure; most end up octahedral, though some are also cubic or irregular.
Because it’s so difficult to distinguish between mined and lab diamonds (even for jewelers), diamond grading institutions inscribe LG or Laboratory-Grown on the rim of lab-grown diamonds, visible at 20-times magnification.
The future of jewelry
The tradition that diamonds are an integral part of an engagement proposal is the result of a highly successful advertising campaign by the De Beers cartel. During the Great Depression, diamond sales slumped. De Beers responded by enlisting Hollywood actors and socialites in a campaign to associate diamond rings with marriage proposals, commissioning portraits of them showing off their new engagement rings, and by running ads showing happy young couples honeymooning above the now-famous slogan ‘A Diamond Is Forever’. In time, it also tried to persuade men that they would need to spend a fixed proportion of their income on a stone to win at love. One later advert, from the 1980s, was captioned ‘2 months’ salary showed the future Mrs Smith what the future would be like’.
De Beers’s campaign worked because people desire signifiers of commitment that are credible and socially sanctioned. Diamond engagement rings suit this purpose because they are beautiful and practical and their symbolism is well-known. While the mine owners benefited most from this arrangement, the high price was a costly – and therefore credible – signal of wealth and commitment and, in an era where this mattered, insurance against breach of promise to marry. Peer pressure and status anxiety reinforced this norm.
Lab diamonds have destroyed this equilibrium. Competition among diamond manufacturers and technological progress in diamond making mean that lab diamonds are indistinguishable from mined diamonds, but cost much, much less – and the price is falling. In 2016, a one-carat near-colorless and very slightly included round brilliant lab-grown diamond cost $5,440, according to diamond analyst Paul Zimnisky; in 2024, the same stone cost $1,325. (The price of an equivalent mined diamond decreased from $6,538 to $5,035.) In the past few years, sales of lab diamonds have started to overtake mined diamonds. A survey by The Knot of nearly 10,000 couples married in 2023 revealed that lab diamonds accounted for 46 percent of engagement rings (compared with 39 percent who opted for a mined diamond), up from 12 percent in 2019.
Diamonds will continue to symbolize engagement – the tradition is now well established – but on its own, the raw material will cease to be a symbol of wealth or sacrifice. Lab diamonds can already be made to be clearer and more colorless than mined diamonds – or, if the wearer desires it, to have a more magnificent color. To complement this, consumers are demanding better workmanship in the cutting and polishing of the stone and in the design and manufacturing of its setting.
The ‘hearts and arrows’ optical pattern, which is present only in round brilliant diamonds that are perfectly cut, is a good example of this trend. Previously, perfectly cut diamonds were extremely rare, and too expensive for most customers. Now, because of strong demand, the International Gemological Institute has begun to note the presence of the hearts and arrows pattern on their diamond grading certificates.
As lab diamonds can be engineered to be more beautiful than mined diamonds, we should expect them to be used more often in fine jewelry. Lab diamonds won’t end conspicuous consumption, but it will be the consumer and not the mine owners who enjoy most of the benefits.
Diamonds in industry
While the beauty of diamonds has made them synonymous with jewelry, most diamonds, mined or lab-grown, are produced for industrial use. Diamonds have excellent physical, optical, thermal, chemical, mechanical, and electrical properties, which makes them useful in manufacturing, construction, mining, medicine, electronics, and other industries. Mined diamonds, being too impure, do not perform as well as lab diamonds when used for industrial purposes. Moreover, lab diamonds can be manufactured to required specifications more reliably and cheaply. In industry, lab-grown diamonds have eclipsed mined diamonds for several decades, and improvements in diamond-manufacturing technology are spurring progress in other fields.

We have not exhausted diamond’s potential. As diamond manufacturing improves, promising new uses of diamonds are being discovered.
Diamond is the hardest naturally occurring material. Its most frequent application is to make hard cutting edges, drill bits, grinding and polishing tools, and abrasives. Diamonds can be embedded in a metal coating or fastened to a metal core to make powerful saws, which are used to cut stone, concrete, asphalt, bricks, glass, ceramics, and metal.

Polycrystalline diamond drill bits, made by fusing together diamond grit under high pressure and high temperature, are indispensable in oil and gas drilling, where they are stronger, faster, and more durable than tungsten carbide, the best alternative. Oil and gas wells often need to be drilled several kilometers deep, and replacing a broken or worn drill bit is a time-consuming process that requires pulling up and breaking apart the entire drill string. Using diamond-tipped drill bits has reduced the need for these replacements, saving money. There are some particularly hard rock formations that would be impossible to drill without them.
Because of their strength, durability, and precision, diamond-tipped drill bits are also used to drill into glass, masonry, ceramics, and teeth. Other applications of diamond that make use of its hardness include grinding and polishing concrete, granite, and marble; drawing wire; and coating other materials to make them more hard-wearing. As materials science advances and creates tougher alloys, polymers, ceramics, and composites, it is almost certain that diamond tools will be needed to cut and shape them.
As an optical material, diamonds have tremendous potential, because they are transparent, dissipate heat quickly, and do not expand much at high temperatures. In particular, diamonds are useful in high-powered lasers, which are used in cutting, welding, sensing, ignition, and medical surgery. A major problem with existing high-powered lasers is that their components are damaged by or deteriorate under high heat, limiting their output. An example of this is the thermal lensing effect, where high and uneven temperatures change how the optical window of the laser bends light. This degrades the focus and alignment of the laser beam. Diamond, however, is an excellent window material, being a good conductor of heat and transmitter of light and having a refractive index that does not vary much with temperature. Diamonds can also be used as heat spreaders to cool down other components in lasers, increasing the maximum power that can be generated.
An ongoing area of research is the use of diamonds as the active laser medium: the component that optically amplifies light. To do this will require diamonds that are larger, purer, and more structurally perfect than what nature can provide and so will depend on advances in diamond-manufacturing technology.
Even more promising, diamond has the potential to be an excellent semiconductor. Diamond has excellent thermal conductivity, because the regularity of its lattice and the strength of its bonds enable heat to be transferred quickly and efficiently. It has a wide band gap – in other words, it requires a high (but not insurmountable) amount of energy to promote one of its electrons into the conduction band. This means diamond can handle higher temperatures and voltages than conventional semiconductors, making it useful not only in devices that operate in extreme conditions (such as engines, radio towers, drilling equipment, spacecrafts, solar panels, and the electricity grid) but also for increasing microchip performance more generally.
Most microchips today are made from silicon, a metalloid that sits one row below carbon on the periodic table and has a thermal conductivity of 1.5 watts per centimeter-kelvin. Diamond, by contrast, has a thermal conductivity of 22 watts per centimeter-kelvin. Over the past five decades, the number of transistors on a microchip has increased at an exponential rate, while the microchips themselves have become smaller. Chip designers have therefore had to contend with the ever-increasing problem of dissipating the heat that is generated. Heat degrades the performance of microchips and limits how tightly transistors can be packed together.
To overcome this problem, manufacturers have lowered the voltage and devoted a large amount of space and energy to cooling and ventilation systems. Because diamond dissipates heat much faster than silicon, diamond-based microchips can be made smaller and operate in more extreme temperatures. On existing silicon-based microchips, diamonds are already being used as heat spreaders.
Diamond also has a wider band gap than silicon (5.45 electron volts vs. 1.1 electron volts) and consequently diamond microchips can operate at higher voltages than silicon microchips. Semiconductors are engineered to precisely control the flow of electricity, but above a certain voltage their electrical resistance breaks down, resulting in an uncontrolled flow of current. Diamond undergoes electrical breakdown at ten millivolts per centimeter, compared to 0.3 for silicon, making it more suitable for high-voltage applications such as power generation and distribution. Furthermore, for a given voltage, less material is needed and so diamond microchips can be made to be smaller.
Compared to other wide band gap semiconductors, electrons and electron holes move quickly through diamonds. This is important, because it means that signals are transported through diamonds faster. A conservative estimate places diamond’s electron and electron hole mobilities at 1,000 cm2/Vs (square centimeters per volt-second) and 2,000 cm2/Vs respectively, but electron mobilities as high as 4,500 cm2/Vs and hole mobilities as high as 3,800 cm2/Vs have been reported. Silicon, for comparison, has an electron mobility of 1,500 cm2/Vs and a hole mobility of 480 cm2/Vs.
Before diamonds can be used in semiconductors, we’ll have to overcome a number of technical challenges. The cost is, at present, prohibitive (despite recent advances, diamond is still about 10,000 times more expensive than silicon) and the diamond substrates required are larger than what can currently be made. Microchips are manufactured from extremely pure, single-crystal wafers, which are thin slices of semiconductor material. While semiconductor fabrication plants can manufacture silicon wafers to have diameters of up to 300 millimeters, at present diamond wafers are limited to ten millimeters if manufactured using the high-pressure, high-temperature process. Chemical vapor deposition diamond wafers can be made slightly larger, but the number of defects in the diamond and hence its utility as a semiconductor is typically worse.
A more pressing problem is doping (that is, deliberately adding impurities to) the diamond to have the required electrical properties. In its pure form diamond is an electrical insulator. To use it as a semiconductor requires incorporating electron-donating impurities, to create an n-type semiconductor, or electron-accepting impurities, to create a p-type semiconductor.
Creating a p-type diamond semiconductor is not hard: boron, the element to the left of carbon in the periodic table, has one fewer electron and hence is electron-accepting. When introduced as an impurity during the diamond-manufacturing process it is readily incorporated into the diamond’s lattice.
Creating an n-type diamond semiconductor is harder. While nitrogen, the element to the right of carbon in the periodic table, has one surplus electron, the energy required to release this electron to the conduction band is extremely high. Phosphorus, which is one period below nitrogen, is a more promising candidate, but because phosphorus is larger than carbon, incorporating it into the tightly packed diamond lattice is not easy. The energy required to release the surplus phosphorus electron is also high, though the situation is not as bad as it was with nitrogen. When such an n-type diamond semiconductor is used at high temperatures, this is less of a problem, as the required activation energy is readily available. However, for semiconductors intended to be used at room temperature, a more suitable electron donor must be found. This is an open problem.
Not unconquerable
Nature takes billions of years to produce diamonds. Deep in the Earth’s mantle, the intense heat and high pressure slowly crystallizes carbon-containing fluids into diamonds. Volcanic eruptions transport these diamonds to the surface. Mining them is a dirty business – diesel-powered diggers excavate 1,000 tons of earth for every carat that is dug out of the ground. Most mined diamonds are ugly, discolored, and impure.
In the lab, diamonds can be made to be purer, more beautiful, and more durable than what nature can achieve in less time and with fewer resources. If desired, specific impurities can be added to modify their optical, mechanical, and electrical properties. Initially, lab diamonds were manufactured using the high-pressure, high-temperature process, which was inspired by the method by which diamonds form in the Earth. Now they are also made using chemical vapor deposition, which has never been observed in nature.
In Western civilization, a pernicious belief has taken hold, that what is ‘natural’ is good and what is man-made is inferior or harmful. Wordsworth, in his poem ‘The Tables Turned’, expresses it best:
Sweet is the lore which nature brings
Our meddling intellect
Mis-shapes the beauteous forms of things: —
We murder to dissect.
But human intellect and the scientific method are the foundations of our prosperity. Nature is not a superior craftsman: it is our resource, and we can and should improve upon it.
In materials science, this has been obvious for a long time: steel, plastic, polyester, concrete, ceramics, and glass are unsurpassed by any natural substance. Lab diamonds are a further testament to this.