The story behind humanity’s greatest environmental success is too rarely told and too often taken for granted. This is how humanity fixed the ozone layer and why it matters.
When it comes to stories of progress, there aren’t many environmental successes to learn from. We’ve seen massive improvements in many human dimensions in recent decades – declines in extreme poverty; reductions in child mortality; increases in life expectancy. But most metrics that relate to the environment are moving in the wrong direction. Although there are some local and national successes – such as the large reductions in local air pollution in rich countries – there are almost none at the global level.
Yet there is one exception: the ozone layer. Humanity’s ability to heal the depleted ozone layer is not only our biggest environmental success, it is the most impressive example of international cooperation on any challenge in history.

Subscribe for $100 to receive six beautiful issues per year.
The emerging ozone hole: how did we realise there was a problem?
Let’s first lay out some of the basics about ozone. Ozone (O3) is present in Earth’s atmosphere at multiple levels. Where ozone is present in the atmosphere determines whether it is referred to as ‘bad’ or ‘good’ ozone. Some ozone is formed at ground-level through reactions with local air pollutants emitted from vehicle exhausts, industrial processes and chemical solvents. This is so-called ‘bad ozone’ because it can damage your health, especially if you are young, old or have underlying respiratory problems. It can cause chest pain, breathing problems, inflammation of the airways and long-term tissue damage in the lungs. You don’t want to be breathing in ozone.
But we also have ozone very high in the atmosphere – around 15 to 35 kilometres above the surface, in the stratosphere. This is the so-called ‘good ozone’. Although good and bad ozone are identical molecules, the position of good ozone in the stratosphere means that it plays a crucial role in absorbing dangerous ultraviolet (UV-B) radiation from the sun. This protective layer of ozone guards humans from skin cancer, sunburn and blindness and is crucial in protecting other lifeforms as well. What this means is that we want to remove ground-level ozone, but we definitely don’t want to remove it in the stratosphere.
Paul Crutzen – who sadly died earlier this year – was a Dutch atmospheric chemist and the first to raise concern that humans were affecting the chemistry of the stratosphere. In the 1960s, scientists were beginning to understand the reactions that determined the photochemistry of the upper atmosphere. At the time, many scientists used models that were built around the interactions between OH radicals and ozone molecules.
But Crutzen was unconvinced by them: he concluded that these reactions could not explain the concentrations of ozone high in the stratosphere, and suggested there were other factors at play; that interactions between chemicals, such as nitrogen compounds, and light were also occurring in the stratosphere. Unfortunately there had been no measurements of stratospheric nitrogen to prove it.
But within a year he had the data he needed. Researchers had figured out how to measure the density of nitrogen compounds (HNO3) in the stratosphere, using solar spectrum experiments on high-altitude balloons. Crutzen not only realised that nitrogen could react with ozone in the stratosphere, but that human emissions of nitrogen compounds could be affecting these reactions. Humans were producing nitrous oxide (N2O) through multiple processes such as crop fertilizers, rocket motors and combustion engines. And these emissions could reach the stratosphere, react with ozone (O3) and cause it to break down into oxygen (O2). UV radiation provided a perfect catalyst for these reactions.
Crutzen’s biggest concern of the time was that the introduction of large stratospheric fleets of supersonic aircraft – which emit nitrogen compounds – would have a large impact on the ozone layer. He thought it could be a major environmental problem, in contrast to other researchers who had concluded that these fleets would have little impact on ozone photochemistry. His frustration was palpable; he described how he had reacted to other scientists who dismissed his concerns at a conference: ‘Somewhere in the margin of this text I wrote “Idiots”’.
Only a few years on, the scientists Frank Rowland and Mario Molina proposed that human emissions of chlorine substances might have exactly the same impact. These substances – the most well-known ones being chlorofluorocarbons (CFCs) – were being widely used in refrigerators, freezers, air conditions, aerosol sprays and in industrial production. By measuring concentrations of chlorine molecules throughout the lower atmosphere, they realised it was clear that they were not breaking down: the amount in the atmosphere was almost exactly equal to the cumulative production to date. The chemical inertness that made them great for technology was also preventing them from breaking down in the lower atmosphere.
So, Rowland and Molina modelled the possible sources and sinks of these compounds to work out where they might eventually be lost. They recognised that the only possible sink was in the stratosphere, where UV radiation would break the chlorine atoms free, allowing them to react with ozone, and break it down. The evidence was growing: humans were depleting the ozone layer through their use of these depleting substances.
Unsurprisingly, many industrial players tried to discredit their work. The fact that there was not yet experimental evidence made this easier – they could dismiss it as pure speculation. The chairman of DuPont – the largest global manufacturer of CFCs – said the theory was: ‘a science fiction tale . . . a load of rubbish . . . utter nonsense’. The leading producers formed the ‘Alliance for Responsible CFC’ to coordinate their efforts; they launched intense PR campaigns discrediting the theory of ozone depletion.
Crutzen, Rowland and Molina would eventually get the credit they deserved, however; in 1995 they won the Nobel Prize in Chemistry for their work.
Forming the world’s most successful international agreement
Scientific consensus on the ozone depletion problem continued to emerge, and relatively quickly. Rowland and Molina first shared their hypothesis in 1974. By 1976 the United States National Academy of Sciences released a report concluding that their hypothesis was strongly supported by the scientific evidence. By 1979, NASA was tracking the concentration of ozone in the stratosphere using Total Ozone Mapping Spectrometer instruments. Year-on-year they saw a continued decline.
Unlike the pace of scientific research however, the political response lagged behind. Several countries – the US, Canada and Norway – only banned the use of CFCs in aerosol sprays, in 1978, after many consumers moved away from using them voluntarily. These piecemeal contributions were far from enough. Progress stalled for many years with strong resistance from the big industry players such as DuPont and more resistance to environmental regulation came from the Reagan administration. Anne Gorsuch, the US’s first female head of the Environmental Protection Agency, dismissed ozone depletion as just another environmental scare.
Things changed quickly in the mid-1980s for both scientific, political and economic reasons. NASA now had several years of data showing a depletion of stratospheric ozone. This culminated in a major report in 1985 where it laid out the scientific evidence for humanity’s impact on the ozone layer. But the biggest scientific game-changer was the discovery of the ozone hole over Antarctica. It seemed to come from nowhere. Scientists had been collecting long-term datasets from ground-based stations in Antarctica for years. In 1981, there were small hints, but by 1983 the picture was clear. Below, you can see the dramatic image captured in 1983. The British Antarctic Survey scientists, Farman, Gardiner and Shanklin, didn’t publish their results in Nature until 1985 – the finding was so sudden, and implications so big, that they had to be sure it wasn’t simply a measurement error. The visual imagery of a growing ozone hole put pressure on governmental and industrial actors to take action.
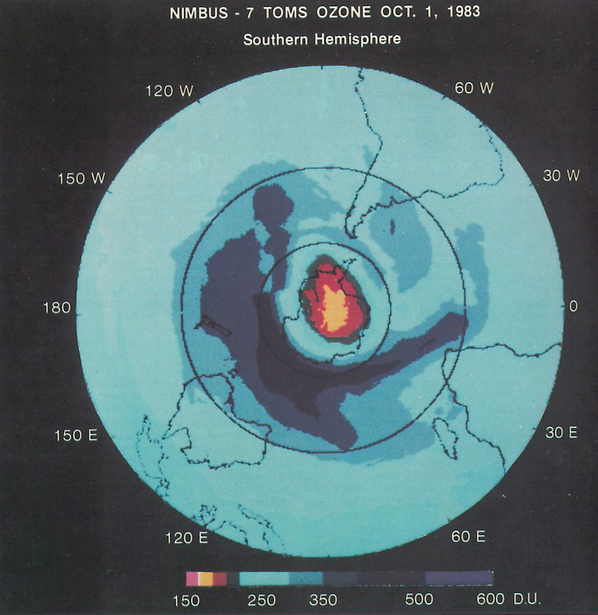
This finding couldn’t have come at a better time politically. William Ruchelshaus had just taken over from Anne Gorsuch as head of the US EPA. He was already much more concerned about the ozone problem, and the increased public concern from the ozone hole discovery gave him even more leverage to push for domestic and international action. The US, as an industrial leader, was in prime position to lead international efforts. In 1985, the Vienna Convention (also known as the Vienna Convention for the Protection of the Ozone Layer) was born. Twenty countries, including most of the major CFC producers, signed up to coordinate international regulations on these ozone-depleting substances.
The position of some industry players also shifted quickly. DuPont’s patent on CFC technologies had expired. With real prospects of tough regulations, it quickly shifted gears to turn risk into opportunity. It announced that the barrier to safer alternatives was price, not chemistry. It said it could develop alternatives within years if there were the regulatory and market incentives to do so. It went for a first-mover advantage.
The members of the Vienna Convention formed their first protocol, called the Montreal Protocol. Forty-three countries signed on in 1987, agreeing to phase out ozone-depleting substances from 1989 onwards. This first group of countries was mostly limited to richer countries that were the main industrial producers of the time – US, Canada, Japan, most of Europe and New Zealand. Their aim was to halve global production of these substances by 1999, from their 1986 levels. What people often miss, however, is that the first Montreal Protocol would have been totally inadequate to fix the problem. The reduction target was weak, and the list of substances that were included was incomplete. If we had stuck to the target, the hole in the ozone layer would have continued to grow.
The success of the Vienna Convention lay in its increasing ambition over time. Regulations became tighter as more evidence emerged of the depletion of the ozone layer and the gases that were causing it. The deadline to phase out the production of ozone-depleting gases continued to be brought forward. More countries joined. By the turn of the millennium, 174 parties had signed on. In 2009, it became the first of any convention to achieve universal ratification.
Consumption of ozone-depleting substances has fallen by 99.7%
The success of this international effort was truly stunning. Before the first protocol came into action in 1989, the use of ozone depleting substances continued to increase. But the phaseout that followed was rapid. Within a year, consumption fell 25% below its 1986 levels. Within a decade the levels had fallen by almost 80% (far beyond the initial target of the Montreal Protocol of a 50% reduction). As of today, their use has fallen by 99.7% compared to 1986.
We have, effectively, phased out these substances completely, with only a few granted exemptions. Many companies replaced them with less damaging alternatives, such as hydrofluorocarbons (HFCs). The problem with some ozone depleting substances was that they contained chlorine or bromine, highly reactive elements that could ‘steal’ an oxygen atom from ozone (O3) to form ClO or BrO, which would cause ozone to break down. Since HFCs do not contain chlorine or bromine, they don’t have this depleting effect. The downside to HFCs is that they are a potent greenhouse gas and still contribute to climate change.
Chart showing the change in the consumption of these ozone-depleting substances relative to the index year, 1986 (which is set to a value of 100).
Is the ozone hole recovering?
Although the global response to ozone depletion was fast, it will take the ozone layer much longer to recover. But a full recovery is possible. We know this because we can measure concentrations of ozone in the stratosphere. NASA has been monitoring ozone concentrations and the size of the ozone hole in their Ozone Watch division since the late 1970s.
We measure ozone concentrations using a metric called ‘Dobson Units’ (DU). A Dobson Unit is the number of molecules of ozone that are needed to create a layer of ozone that is 0.01 millimetres thick under particular temperature and pressure conditions. An area where the DU drops to 100 or less would signify an ‘ozone hole’.
Ozone concentrations in the stratosphere were falling rapidly through the late 1970s and 1980s. Concentrations halved within a decade, reaching dangerous levels below 100 DU. The ozone layer, which had been protecting us from the dangerous effects of UV-B light, was breaking down. But our global efforts began to succeed. As we rapidly reduced our emissions, we stabilised concentrations of ozone. We were no longer depleting the stratosphere of this precious gas.
Chart showing the concentrations of stratospheric ozone, measured in Dobson units.
What impact did this have on the size of the ozone hole? Since there is a delay until the effects are reflected in the size of the ozone hole, the opening of the Antarctic hole was still observed through the 1980s. By the late 1990s it had stretched to 30 million kilometers squared – three times the size of the United States. But the fruits of our efforts were eventually visible. Since the late 1990s, the size of the ozone hole has stabilised. In 2018, the NASA Aura Program published its first results to show clear initial signs of recovery.
Restoring decades of damage to the ozone layer will take time. Global concentrations of ozone are not expected to return to their 1960 levels until the mid-21st century. Antarctica, where ozone depletion has been most severe due to very low temperatures, is expected to recover much more slowly. There, we might be waiting until the end of the century. As long as we stick to our phaseout of ozone depleting substances, there is no reason to believe that things will not continue to improve. But even if we have to wait some time, it is a wait that is worthwhile.
Chart showing the size of the Antarctic ozone hole, measured in square kilometres (km2).
Can we tackle other environmental problems in the same way?
Our efforts to tackle other environmental problems have not been quite so successful. Can we extrapolate any of the lessons from the task of fixing the ozone layer to other challenges, such as climate change?
There are of course many similarities: ozone depletion and climate change are shared, global problems. Unlike air pollution where local residents are impacted by local emissions, it is the entire global population that is impacted by ozone depleting substances and greenhouse gas emissions. This is because these gases disperse easily across the globe; they are known as ‘well-mixed’ gases. The need for international coordination on both issues is therefore obvious.
We can also learn from the ramping up of efforts over time. The ambition of the first Montreal Protocol in the 1980s was far too weak to solve the problem. Although it was better than ‘business as usual’, the target would have meant that the ozone hole would have continued to expand. Our efforts were only successful because we continued to raise the standards of regulation over time. Climate policy is in a similar position today, and has been for a long time. Current commitments from countries have us on course for a warming of 3℃ by 2100, well beyond the UN target of keeping warming below 2℃. If we want to achieve our target, we need to step up our ambitions – fast.
Yet there are important differences between these problems. While both are global problems, their impacts are not felt equally across the world.
Ozone depletion is worse at higher latitudes, where the air is colder, which is part of the reason why ozone holes form over Antarctica and the Arctic. Richer countries, such as those across Europe and North America, are not only located where ozone depletion is higher, but their populations are also more likely to be vulnerable to risks such as skin cancer, due to skin colour. There was therefore strong incentive for the world’s largest producers of ozone-depleting substances to take action. A headline piece in the New York Times in 1986 warned of millions of excess skin cancer cases in the coming decades as a result of ozone depletion. The fact that the largest producers had the most to lose probably accelerated efforts to fix it.
This is not the case with climate change: those at greatest risk of climate impacts are typically the world’s poorest, and do not have the resources to adapt. Those who contribute least to greenhouse gas emissions are those who have the most to lose. The incentives to preserve the status quo are flipped.
Another big difference between the problems of ozone depletion and climate change is that ozone depletion was an industry-specific problem while climate change is an economy-wide one. It was much easier to find substitutes for the substances we used in refrigerants and aerosols than it is to reshape our whole economy. We didn’t need to stop chilling food or propelling deodorant, we just needed to find a new way of doing it.
But our lives, industry, transport, electricity sources, and food systems have been built on carbon-emitting fuels. Many of these infrastructural systems have a lifetime that lasts decades. Completely revamping the way we travel, grow food or produce energy is not something that can happen overnight. The good news is that the cost of low-carbon energy sources is plummeting, which should help to make this the new default choice. But reshaping the systems that economies across the world stand on was never going to be as straightforward as replacing the gases in our refrigerators.
Despite the fact that tackling climate change will be more difficult, I still think there are important lessons to learn from the ozone success story. We can tackle real global problems. We can involve every country in this process. And we can take action quickly when we’re running up against time. Perhaps the fact that we rarely talk about the ozone layer anymore is a testament to our success in tackling it, but it serves us well to remind ourselves that we are capable of cooperating on such global problems, which is why I like to return to and relive the story every so often.
